Common Drug a ‘Silver Bullet’ for Severe COVID-19 Patients, Israeli Scientists Find
Study led by Hebrew University shows fat-lowering drug Tricor (fenofibrate) removes need for respiratory support for hospitalized patients within five to seven days
A readily available drug used to treat high lipid levels in the blood has been shown to “dramatically” help in the treatment of severe COVID-19 cases, according to a new study.
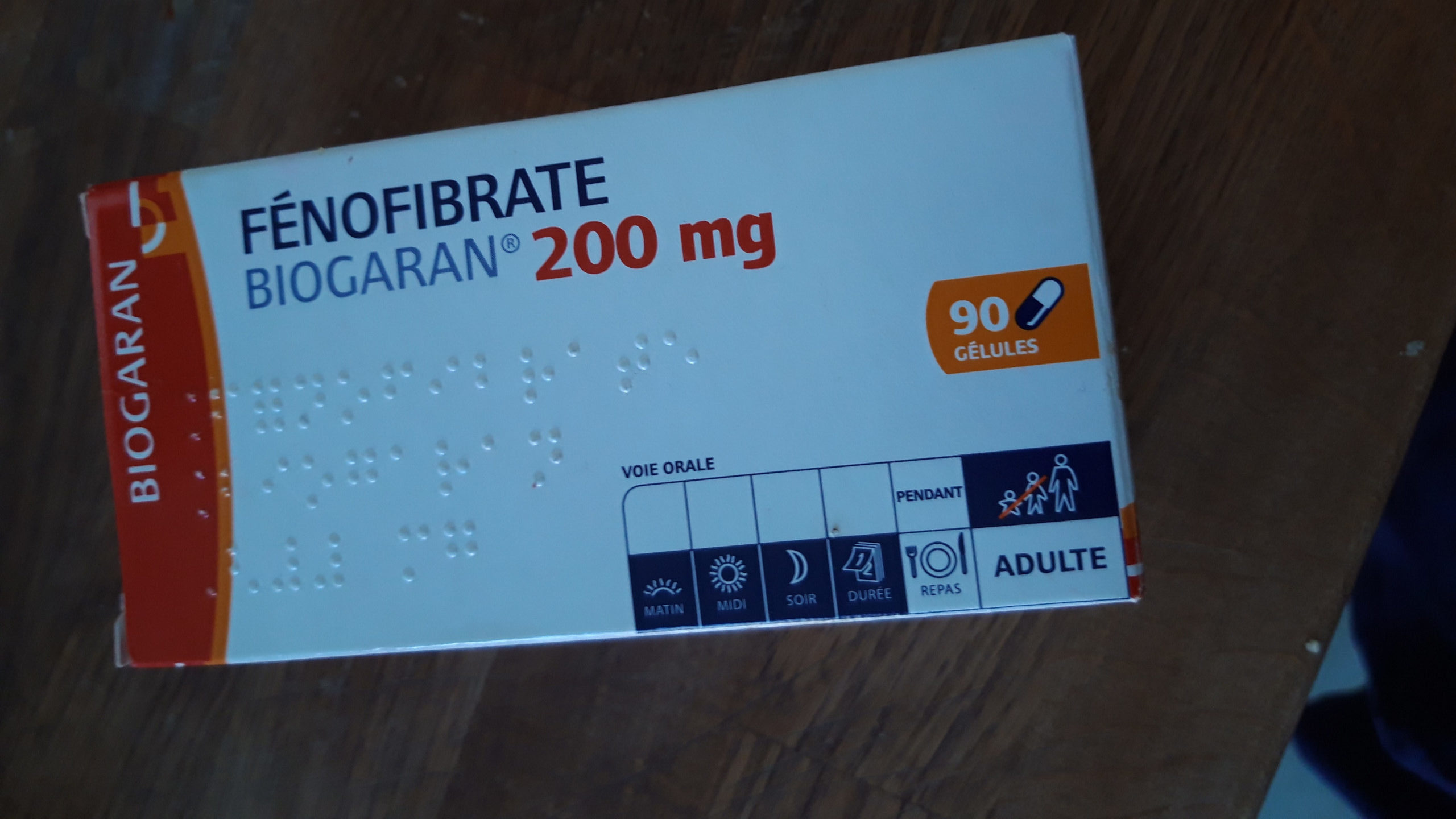
In the investigator-initiated interventional open-label clinical study, a research team led by Prof. Yaakov Nahmias, director of the Grass Center for Bioengineering at the Hebrew University of Jerusalem, administered 145 mg/day of TriCor (fenofibrate) to 15 patients hospitalized with severe COVID-19 over a period of 10 days and repeatedly monitored their disease progression.
“What we saw is that their inflammatory markers dropped very, very fast,” Nahmias told The Media Line. “Within 48 hours of receiving the drug, they simply didn’t have inflammation at all. There was no overreaction of the immune system as we usually expect to see in severe patients.”
The interventional clinical study was carried out on patients at Israel’s Barzilai Medical Center in the southern city of Ashkelon, with support from Abbott Laboratories. It was recently published on Research Square and is currently under peer review.
Within 48 hours of receiving the drug, they simply didn’t have inflammation at all. There was no overreaction of the immune system as we usually expect to see in severe patients
The clinical outcomes of the study were dramatic: 14 out of 15 patients, or 93.3%, no longer required respiratory support after five to seven days of treatment, compared to 28.5% of patients in a historical control group. The final patient also eventually recovered and ended up being released some time later.
Though preliminary, the findings could very well indicate that TriCor is a “silver bullet” for the treatment of the virus, Nahmias said.
This holiday season, give to:
Truth and understanding
The Media Line's intrepid correspondents are in Israel, Gaza, Lebanon, Syria and Pakistan providing first-person reporting.
They all said they cover it.
We see it.
We report with just one agenda: the truth.


“Altogether, this is a very strong result,” he affirmed. “Usually, it takes several weeks to treat severe COVID-19 patients.”
“It’s highly statistically significant even though it’s a small study because we took markers every two days and we could actually show a dramatic effect on the time course of the disease,” he said.
All of the patients were above the age of 47, with the median age being 64. In addition, some had multiple comorbidities, such as diabetes, high blood pressure and obesity, among others.
Researchers recently found that COVID-19 causes an abnormal accumulation of lipids, which lead to severe inflammation in a process known as lipotoxicity. Fenofibrate, an FDA-approved drug since 1975, is typically used to treat symptoms of cholesterol and lower triglycerides in the blood.
While Nahmias and his team focused on severe cases, he believes fenofibrate could eventually also be used to treat moderate or mild cases and help speed up recovery.
The drug could represent a breakthrough in COVID treatments and a significant improvement over other medications that have until now been experimentally used in severe cases – such as hydroxychloroquine and Ivermectin – and are known to have serious side effects.
“Patients have been taking fenofibrates for decades,” Nahmias explained. “What we’re suggesting here is a 10-day treatment and it’s a very, very safe drug. Its side effects are mild even in long-term treatments.”
Prof. Shlomo Maayan, head of Barzilai Medical Center’s Infectious Disease Division, coordinated the study.
“We observed a fast recovery of those patients, faster than we expected,” Maayan told The Media Line. “All needed [respiratory] support, and all, after five days of treatment, did not need this support any longer.”
While the early results are very promising, he said, a double-blind study must be completed before the drug becomes a more widely available treatment.
We observed a fast recovery of those patients, faster than we expected
Phase III clinical trials are already underway in multiple corners of the globe, including at Barzilai Medical Center in Israel. Another major trial is being coordinated by the University of Pennsylvania, which has clinical centers in South America, North America and Europe.
Though the trials are expected to take six months to complete, according to Nahmias, physicians can already decide to use TriCor on a case-by-case basis for emergency purposes, pursuant to local laws.
“Physicians can already decide to treat with fenofibrates based on early clinical data,” he said. “That said, approval would definitely require a placebo-controlled study and we think it’s going to take several more months until the data is available.”